Nuclear Magnetic Resonance (NMR) spectroscopy
It is a powerful analytical technique used for determining the structure of organic compounds by studying the interaction of atomic nuclei with an external magnetic field.
It provides detailed information about molecular structure, functional groups, molecular dynamics, and interactions.
It is widely used in chemistry, biochemistry, and pharmaceutical sciences.
Quantum Number & Their Role in NMR
Quantum numbers describe the behaviour and properties of nuclei.
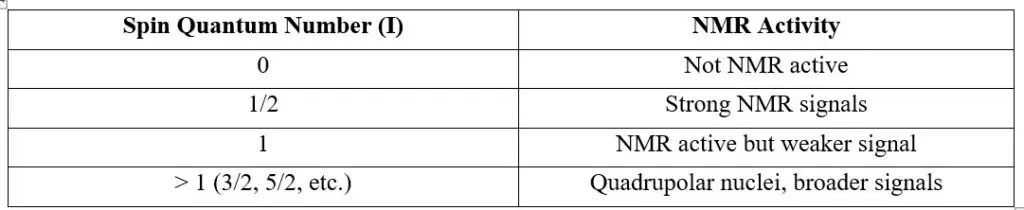
1. Nuclear Spin Quantum Number (I) and NMR Activity
The nuclear spin quantum number (I) represents the intrinsic angular momentum of a nucleus. This property depends on the total number of protons and neutrons (nucleons) in the nucleus.
The presence of spin allows nuclei to interact with an external magnetic field, making them detectable in NMR.
Selection Rule for NMR:
- Only nuclei with I ≠ 0 exhibit NMR signals.
- If I = 0 (even number of protons and neutrons), the nucleus has no magnetic moment and does not participate in NMR.
- If I > 0, the nucleus has a magnetic moment and is NMR-active.
2.Magnetic Quantum Number (mI) and Energy Level Splitting
Once we place an NMR-active nucleus in a strong external magnetic field (B0B_0B0), its spin states split into multiple energy levels due to the Zeeman effect. The number of these energy levels is determined by the magnetic quantum number (mIm_ImI), which takes values from +I to -I in integer steps.
For a spin-1/2 nucleus (I = 1/2),
There are two possible spin states:
- mI=+1/2 (aligned with the magnetic field, lower energy state)
- mI=−1/2 (opposed to the magnetic field, higher energy state)
Principle of NMR
- When nuclei placed in a strong magnetic field, certain nuclei absorb electromagnetic radiation at specific radio frequencies.
- The absorption occurs due to the transition between nuclear spin states, which is influenced by the external magnetic field and the surrounding electron environment.
- The energy difference (ΔE) between these spin states is given by the Larmor equation:
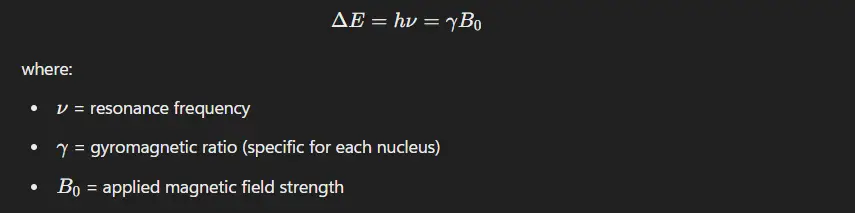
Instrumentation in NMR
Major Components of NMR Spectroscopy
1.1. Magnet System
- The superconducting magnet generates a strong and uniform magnetic field (B₀) required for nuclear spin alignment.
- Field strength ranges from 200 MHz to 1000 MHz (Tesla scale: 4.7T – 23.5T).
- Liquid helium and nitrogen are used for cooling the superconducting coils.
→ Provides a stable magnetic field to ensure accurate frequency resonance.
→ Higher field strength improves sensitivity and resolution.
- The radiofrequency (RF) transmitter generates pulses to excite the sample nuclei.
- The probe head contains RF coils that emit and detect RF signals.
- The RF frequency is adjusted based on the type of nucleus being studied (e.g., ¹H, ¹³C, ³¹P).
Importance:
→ Determines the energy transfer for resonance excitation.
→ Custom probes allow analysis of different nuclei (multinuclear NMR).
- Sample tubes: Typically 5 mm in diameter, made of high-purity quartz or glass.
- Sample spinning: Increases homogeneity and improves spectral resolution (especially in solid-state NMR).
- Cryoprobes: Enhance sensitivity by cooling the detection coil and preamplifier.
Importance:
→ Ensures a homogeneous magnetic field across the sample.
→ Cryoprobes improve the signal-to-noise ratio (SNR).
1.4. Receiver and Detection System
- The RF receiver detects the NMR signal as a free induction decay (FID).
- The signal is amplified and converted into a frequency-domain spectrum using Fourier Transform (FT-NMR).
- The pre-amplifier minimizes noise and enhances weak signals.
Importance:
→ Converts time-domain signals to frequency-domain for analysis.
→ Improves detection of low-abundance nuclei like ¹³C.
1.5. Data Processing and Computer System
- Analog-to-digital converter (ADC): Converts FID into a digital signal.
- Fourier Transform (FT) algorithm: Converts raw data into spectra.
- Software processes chemical shifts, coupling constants, and integration values.
Importance:
→ Provides accurate chemical shift and multiplet analysis.
→ Enables 2D-NMR (COSY, NOESY, HSQC, HMBC) for structural determination.
Working of NMR Spectroscopy (Step-by-Step)
Step 1: Sample Preparation
- Dissolve the sample in a deuterated solvent (CDCl₃, D₂O, etc.).
- Place the sample in a 5 mm NMR tube and insert it into the probe.
Step 2: Application of Magnetic Field (B₀)
- • The superconducting magnet aligns nuclear spins in either parallel (low energy) or antiparallel (high energy) states.
Step 3: RF Pulse Excitation
- A short RF pulse is applied, flipping the nuclear spins into a higher energy state.
Step 4: Relaxation and Signal Detection
- When RF is turned off, nuclei relax back, emitting RF signals.
- These signals are recorded as a free induction decay (FID).
Step 5: Fourier Transform (FT-NMR)
- The FID signal is converted into a frequency-domain NMR spectrum.
- Peaks represent chemical environments of nuclei in the sample.
Solvent Required In NMR
Requires a deuterated solvent to avoid interference from hydrogen (¹H) signals in the sample.
Deuterated solvents contain deuterium (²H, D) instead of protium (¹H), which does not produce signals in the usual ¹H NMR spectrum.
D₂O, CDCl₃, C₆D₆, CD₃OD
Chemical Shift in NMR Spectroscopy
Chemical shift (δ\deltaδ) in NMR is the relative resonance frequency of a nucleus compared to a reference compound, typically Tetramethylsilane (TMS, (CH₃)₄Si).
It provides valuable information about the electronic environment of nuclei in a molecule.
• Measured in parts per million (ppm) for easy comparison across different spectrometer frequencies.
• Reference Standard: TMS is assigned a chemical shift of 0 ppm since it is highly shielded.
Factor Influencing NMR
Chemical shift is primarily affected by the electronic environment surrounding the nucleus.
(A) Electronegativity of Nearby Atoms
- Electronegative atoms (e.g., O, N, F, Cl, Br) withdraw electron density, leading to deshielding.
- This causes the nucleus to experience a stronger external magnetic field, shifting the signal downfield (higher δ value).
- Example:
o Methane (CH₄) → 0.2 ppm (shielded)
o Methyl chloride (CH₃Cl) → 3.1 ppm (deshielded by Cl)
o Methyl fluoride (CH₃F) → 4.3 ppm (more deshielded than CH₃Cl)
📌 Trend: More electronegative atoms = Higher chemical shift (downfield shift).
(B) Hybridization of Carbon Atoms
- Hybridization affects electron density and shielding:
o sp³ hybridized carbon (alkanes) → More electron density (shielded) → Lower δ (0-3 ppm)
o sp² hybridized carbon (alkenes, aromatics) → Less electron density (deshielded) → Higher δ (5-8 ppm)
o sp hybridized carbon (alkynes) → Unique shielding effect → Lower δ (2-3 ppm)
(C) Anisotropic Effects (π-Bonding Systems)
- Magnetic anisotropy occurs in π-electron systems, where circulating electrons create secondary magnetic fields that alter the chemical shift.
- • Examples:
o Aromatic Rings (Benzene, Phenyl Groups)
Strong deshielding effect due to ring current → 7-8 ppm
o Alkenes (-C=CH-)
Deshielding of vinylic protons → 4-6 ppm
o Alkynes (-C≡C-H)
Shielding due to ring current → 2-3 ppm
📌 Aromatic and alkene protons shift downfield due to deshielding, while alkyne protons shift upfield due to shielding.
(D) Hydrogen Bonding
- Hydrogen bonding can cause deshielding by pulling electron density away from the proton.
- The chemical shift varies with solvent, temperature, and concentration.
(E) Solvent Effects
- Solvents influence chemical shifts by solvating the sample and modifying electron density.
- Polar solvents (e.g., DMSO-d₆, CD₃OD) may cause downfield shifts due to strong solute-solvent interactions.
- Non-polar solvents (e.g., C₆D₆) have minimal effect on chemical shifts.
(F) Temperature Effects
- Increasing temperature reduces hydrogen bonding, causing upfield shifts (lower δ).
- Decreasing temperature enhances deshielding, shifting signals downfield (higher δ).
📌 Example:
• OH proton in ethanol at room temperature: ~3.5 ppm
• OH proton in ethanol at higher temperature: ~2.5 ppm (weaker hydrogen bonding)
