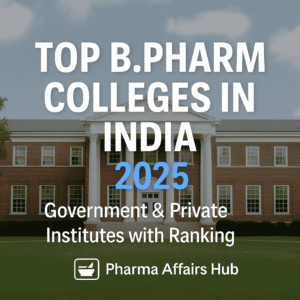
Mass Spectrometry
Mass Spectrometry (MS) is an analytical technique used to identify the composition, structure, and molecular weight of compounds by measuring their mass-to-charge ratio (m/z).
It is widely used in pharmaceuticals, forensics, environmental studies, and biological research to analyze unknown substances and verify sample purity.
Basic Principles of Mass Spectrometry
Organic Molecules are Bombarded with electron → Converted into highly energetic +ve charged ions → breakup into smaller ions → formed ions are separated by deflection in magnetic field a/c to mass/charge ratio.
The fundamental principle of MS involves:
1. Ionization – The sample is converted into gaseous ions.
2. Acceleration– Ions are accelerated by an electric field, imparting them with kinetic energy.
3. Separation – Ions are separated based on their mass-to-charge ratio (m/z).
4. Detection – Separated ions are detected and analyzed based on their intensity.
Ionization:
• The sample (solid, liquid, or gas) is introduced into the mass spectrometer and converted into ions.
• Different ionization techniques (e.g., Electron Ionization, Electrospray Ionization, MALDI) are used based on the nature of the sample.
• Ionization can be soft (minimal fragmentation, e.g., ESI, MALDI) or hard (extensive fragmentation, e.g., EI, CI).
• The formed ions can be single-charged or multiply charged, which affects their mass spectrum.
2. Acceleration:
• The generated ions are accelerated using an electric field, ensuring they have uniform kinetic energy.
• The focusing system (electrostatic lenses) directs these ions into the mass analyzer for separation.
3.Mass Analysis (Separation of Ions):
• The ions travel through a mass analyzer, which separates them based on their m/z ratio.
• Different analyzers operate on different principles:
- Time-of-Flight (TOF): Ions with lower mass travel faster than heavier ions.
- Quadrupole: Uses oscillating electric fields to selectively filter ions based on m/z.
- Magnetic Sector: Ions bend in a magnetic field, and the curvature depends on their mass.
- Ion Trap: Traps ions using electric/magnetic fields and releases them sequentially.
- Orbitrap & Fourier Transform (FT-MS): Measures ion oscillation frequencies in an electric field to determine their mass with ultra-high resolution.
4. Detection & Signal Processing:
• The separated ions reach the detector, where their arrival is recorded based on intensity and time.
• The detector generates a mass spectrum, which represents the relative abundance of ions as a function of m/z.
• The obtained data is processed to determine molecular weight, isotope patterns, fragmentation pathways, and structural information.
From basic physics, the motion of an ion in an electric/magnetic field follows the equation:

Instrumentation of Mass Spectrometry
1. Sample Introduction System
2. Ionization Source
3. Mass Analyzer
4. Detector
5. Data Processing System
1. Sample Introduction System
- Direct Insertion Probe (DIP): Solid or liquid samples are placed on a probe and inserted into the ionization chamber.
- Gas Chromatography (GC-MS): Used for volatile and thermally stable compounds.
- Liquid Chromatography (LC-MS): Used for non-volatile and thermally labile compounds.
Capillary Electrophoresis (CE-MS): Used for charged biomolecules.
2. Ionization Source
Ionization is the process of converting neutral molecules into charged ions so they can be manipulated in an electric/magnetic field. Different ionization techniques are used depending on the nature of the sample.
(a) Hard Ionization Techniques (Produce extensive fragmentation)
Electron Ionization (EI):
→ High-energy electrons (70 eV) bombard the sample, causing ionization and fragmentation.
→ Commonly used in GC-MS.
Chemical Ionization (CI):
→ A reagent gas (e.g., methane, ammonia) interacts with the sample to produce protonated or deprotonated ions.
→ Produces softer fragmentation compared to EI.
(b) Soft Ionization Techniques (Minimal fragmentation, useful for biomolecules) –
Electrospray Ionization (ESI):
→ Sample is dissolved in a solvent and passed through a charged capillary, creating fine droplets that undergo desolvation, producing gas-phase ions.
→ Used in LC-MS for proteins, peptides, and pharmaceuticals.
Matrix-Assisted Laser Desorption/Ionization (MALDI):
→ A laser pulse ionizes the sample, which is embedded in a matrix.
→ Commonly used for large biomolecules like proteins and polymers.
Fast Atom Bombardment (FAB):
→ Uses a beam of high-energy neutral atoms (e.g., argon or xenon) to ionize the sample.
→ Used for non-volatile and thermally labile compounds.
3. Mass Analyzer
Once the ions are generated, they are separated based on their m/z ratio by the mass analyzer. Different mass analyzers have different principles and resolving powers.
(a) Quadrupole Mass Analyzer
• Uses four parallel rods with oscillating electric fields to selectively allow ions of a specific m/z to pass through.
• Used in LC-MS and GC-MS due to its compact design and rapid scanning ability.
(b) Time-of-Flight (TOF) Mass Analyzer
• Ions are accelerated and travel through a flight tube; lighter ions reach the detector faster than heavier ones.
• Provides high mass accuracy and is commonly used with MALDI-MS.
(c) Magnetic Sector Mass Analyzer
• A magnetic field bends the path of ions based on their m/z ratio.
• Used for high-resolution mass spectrometry (HRMS).
(d) Ion Trap Mass Analyzer
• Traps ions using electric/magnetic fields and sequentially ejects them for detection.
• Includes 3D ion traps and linear ion traps.
(e) Orbitrap Mass Analyzer
• Ions are trapped in an electric field and oscillate around a central electrode.
• Offers ultra-high resolution and accuracy.
(f) Fourier Transform Ion Cyclotron Resonance (FT-ICR) Mass Analyzer
• Uses a strong magnetic field to trap ions and detect their frequencies.
• Provides extremely high resolution and mass accuracy.
4. Detector
The detector records the abundance of separated ions and converts the signal into a mass spectrum.
• Faraday Cup: Simple, low-sensitivity detector that collects ions.
• Electron Multiplier: Detects ions by amplifying their signal through secondary electron emissions.
• Microchannel Plate Detector: Provides high sensitivity and fast response time.
• Photomultiplier Tube: Used for high-speed detection in TOF-MS.
5. Data Processing System
Once the detector records the ion signal, the data is processed using software to generate a mass spectrum.
This spectrum provides information about the molecular weight, isotope distribution, and structural fragmentation of the sample.
Application
- Molecular weight, Formula and element composition.
- Determination, identification of unknown compound.
- Distinction between cis & trans – isomers.
- Identification of fragmentation pattern.
- Impurity detection.
- Analysis of Protein.

DPSRU Admissions 2025-26: Explore Premier Opportunities in Pharmacy and Allied Health Sciences
NPTEL Pharmaceutical Dosage Forms Course by IIT (BHU) Varanasi: Master Full Guide (2025)

Career Comparison: Clinical Trials vs. Drug Regulatory Affairs – Which Path is Right for You? (2025)
Difference Between Generic and Branded Drugs: A Comprehensive Comparison (2025)

How Generic Drugs Are Approved in India: A Complete Guide (2025)